Implementation guidance for use of the medication resources themselves will be contained in forthcoming use-case specific Implementation Guides.
This implementation guidance applies to four CareConnect profiled resources that contain the same two structures;
- CareConnect-MedicationRequest-1
- CareConnect-MedicationDispense-1
- CareConnect-MedicationStatement-1
- CareConnect-MedicationAdministration-1
Each contain two common structures;
- A STU3 Dosage structure (or subset thereof)
- A reference to a CareConnect-Medication-1 profiled resource
Use of the STU3 Dosage datatype
The STU3 Dosage datatype is detailed in the HL7 FHIR specification and in the Dosage Structure section of this document.
Referencing a CareConnect-Medication-1 profiled resource
A reference to a CareConnect-Medication-1 resource can be implemented in three ways;
- As an internal reference known as a “contained resource” where the resource is embedded inside the parent resource. This pattern is strongly discouraged in FHIR-based systems. It is used as the basis of examples here only to give clear, compact illustrative examples.
- As an internal reference to a resource defined elsewhere within a FHIR bundle. Use this when an implementation requires the use of a bundle, for example NHS Digital Transfer of Care.
- As an external reference to a RESTful API that would return a resource. This is the preferred pattern for referencing in production systems.
Contained resource
FHIR bundle
External reference
Use of the different dm+d concepts
All references to medication must use the NHS standard of dm+d, which is published as an independent terminology product using XML format data as well as being included in the SNOMED-CT UK Drug extension.
The dm+d code used within a medication resource will vary depending on the level of detail required by the prescriber. The most generic instruction would specify a Virtual Therapeutic Moiety (VTM) plus a dosage instruction. The most specific instruction, for the purposes of prescribing, would specify an Actual Medicinal Product (AMP) plus a dosage instruction.
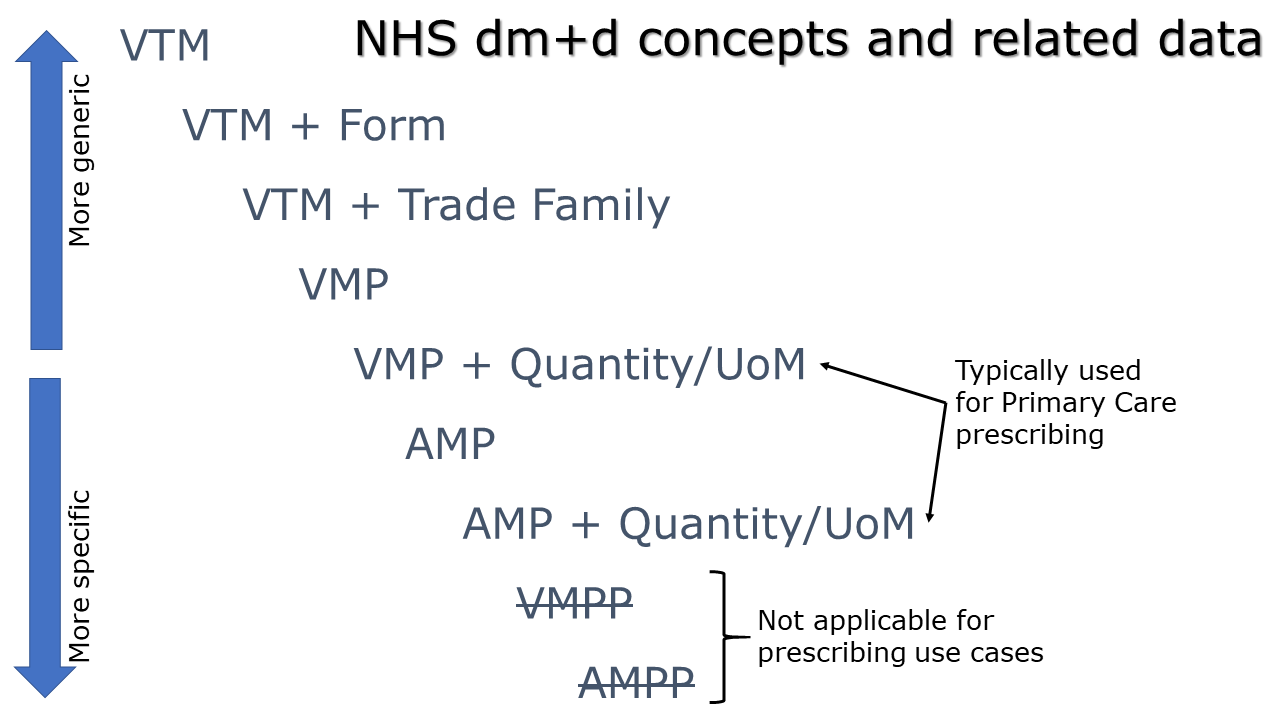
VTM
The most generic representation of a medicine using only a Virtual Therapeutic Moiety (VTM) dm+d concept.
VTM plus Form
A coded form can be defined along with a Virtual Therapeutic Moiety (VTM) dm+d concept where the clinician does not want to be specific with a product-based instruction.
VTM plus Trade Family
The use of a VTM with a Trade Family is a use case not currently supported within FHIR STU3, or through a UK extension. There is currently no part of the Medication resource that is suitable to convey a SNOMED coded Trade Family. This includes the “manufacturer” which is a reference to a CareConnect-Organization-1 structure that is used for organisational contact details such as name, address, ODS code etc. opposed to a SNOMED coded Trade Family.
VMP or AMP
A Virtual Medicinal Product (VMP) or Actual Medicinal Product (AMP) coded concept. Both are pre-coordinated SNOMED-CT coded concepts. A VMP comprises of a medication + strength + form. An AMP may use either the same medication term as the parent VMP or use a 9191801000001103 Trade family (product) for the medication name. An AMP also defines the manufacturer.
